The latest data dump into the U.S. Government’s Vaccine Adverse Events Reporting System (VAERS) happened yesterday (12/3/21) and covers data through 11/26/2021.
There are now 927,740 cases reported to VAERS following COVID-19 shots for the past 11 months, out of the total of 1,782,453 cases in the entire VAERS database filed for the past 30+ years.
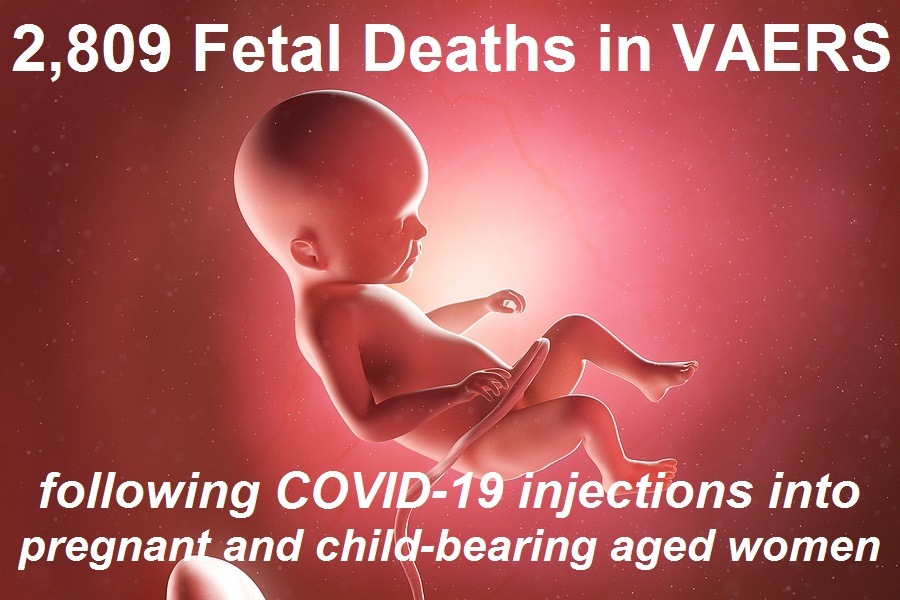
In addition, we found 2,809 fetal deaths following COVID-19 shots injected into pregnant and child-bearing women for the past 11 months.
By way of contrast, using the exact same search parameters in VAERS, but excluding the COVID-19 shots, we found 2,168 fetal deaths following all FDA-approved vaccines for the past 30+ years.
That’s an average of 72 fetal deaths per year following all FDA-approved vaccines for the past 30+ years, compared to what is on pace to be 3064 fetal deaths in 1 year following COVID-19 shots.
That is an 80% increase in fetal deaths recorded in VAERS following the COVID-19 shots. And yet, the CDC and FDA continue to recommend these EUA shots for pregnant women and nursing mothers.
Not only do they recommend these shots for pregnant women, we now have ample evidence that they have known since earlier this year that these shots are dangerous to pregnant women, and causing fetal deaths.
This article will link to publicly available data that shows the CDC, FDA, and Pfizer have known from early on that these shots cause fetal deaths.
With all the links to the publicly available data supplied in this article, there is more than enough information to immediately issue arrest warrants for Rochelle Walensky, the director of the CDC, Janet Woodcock, the FDA director, and Albert Bourla, the CEO of Pfizer, for mass murder and crimes against humanity.