More Evidence the FDA Works for Pfizer as FDA Asks Pfizer to Submit Cancer Drug for Expanded Approval with Zero New Trials
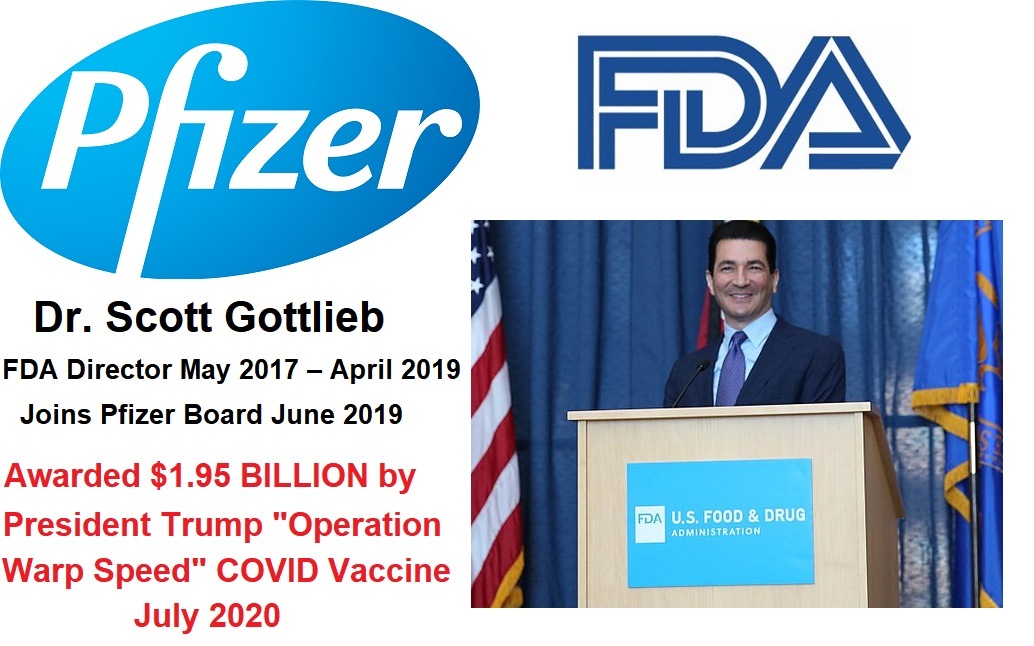
As the alternative media is distracted by the unfounded claim that Pfizer is using gain of function technology to develop new variants of COVID for future vaccines, Pfizer is quietly working together with the FDA to build their portfolio of drugs to make up for the loss of income due to decreasing numbers of people willing to continue getting injected with COVID shots. The evidence that Pfizer controls the FDA continues to mount. We previously reported how Pfizer virtually came out of nowhere to secure the first FDA authorization for experimental COVID shots in 2020, when the two drug companies that had received the most funding and attention to become the first ones to obtain the FDA nod for a new COVID "vaccine" had been Moderna, with their close links to Anthony Fauci and the NIH, and Astrazenca with its close links to Bill Gates. Pfizer's contract with Operation Warp Speed to receive funding for COVID-19 vaccines was different than the other pharmaceutical companies, because it was conditioned on them gaining FDA approval, and it did not include intellectual property rights for the U.S. government. It appears that this opened the door for them to grant an exclusive deal with Israel in exchange for data on how the experimental shots affected people in Israel, effectively making Israel and its citizens lab rats. And a year before Pfizer became a late entry in the contest to get the first FDA authorization for a COVID shot, Dr. Scott Gottlieb joined the Board of Directors for Pfizer in June of 2019, just two months after he finished his term as Commissioner of the FDA under President Trump. This week, Pfizer reported that they expect the sale of their COVID-19 shots and their antiviral Paxlovid to dramatically decrease here in 2023. But what surprised people in the pharmaceutical industry the most about Pfizer's forecast for 2023, was that in a "mysterious" FDA approval that was not reported in the media at the end of 2022, Pfizer received expanded use approval for their breast cancer drug Ibrance, and reportedly, the FDA is the one who approached them to expand the use of this drug! Is there any doubt anymore about who controls the FDA? Pfizer is the largest criminal organization in the world, and they would not be able to continue earning huge profits without their control of the FDA.