Tens of Thousands of Mothers Sue Makers of Tylenol for Pregnancy Use that Led to Babies Born with Autism
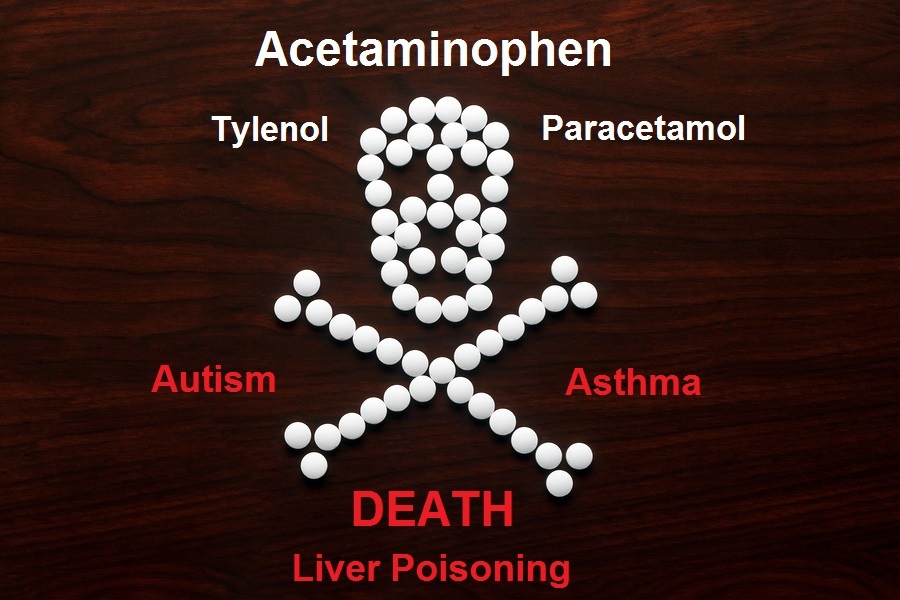
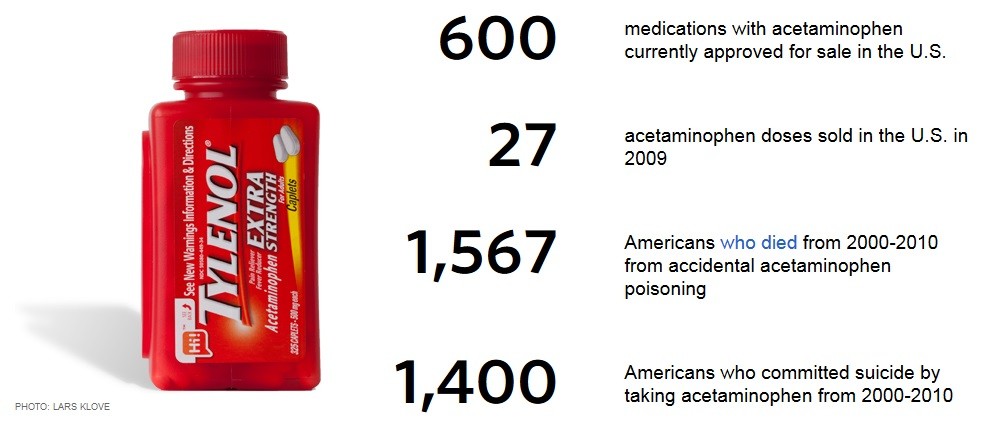
Tens of thousands of mothers are suing the makers of Tylenol for using the popular over-the-counter pain reliever during pregnancy, which resulted in them giving birth to babies diagnosed with autism. Last October, a federal judicial panel consolidated dozens of these lawsuits alleging that acetaminophen, the active ingredient in Tylenol and generic versions of the drug, can cause autism spectrum disorder and attention deficit hyperactivity disorder. The main defendant in the lawsuit is Kenvue, which is the former Johnson & Johnson's consumer health unit that is now a "spin-off" of J&J. Co-defendants in the lawsuit are CVS Health, Rite Aid Corp, Safeway Inc, Target Corp and Walgreens Boots Alliance, which are being charged with failing to warn consumers about the risks of Tylenol. Kenvue has suffered some setbacks in recent weeks in trying to get some of the lawsuits dismissed, with one of the reasons given to dismiss the lawsuits being that the FDA had approved the product and its labels. The judge ruled that the lawsuits can continue. The amount of studies published in the medical journals linking Tylenol taken during pregnancy to babies born with autism is overwhelming. We have been publishing articles exposing the dangers of Tylenol for over a decade now. Pregnant women are the not the only ones who should immediately STOP using this killer drug. NOBODY should be using it. Tylenol is a classic example of how corporate profits from the pharmaceutical drug companies are far more important than patient safety, as millions of lives are sacrificed to keep these drugs on the market. It is also just another example of how the FDA works to protect pharmaceutical companies, and NOT consumers. Tylenol has been on the market for 75 years bringing in annual revenues that exceed $300 million.